Tag: bioequivalence
International Perspectives on NTI Generics: Regulatory Approaches Compared
By Lindsey Smith On 22 Feb, 2026 Comments (5)

NTI generics require strict regulatory oversight due to their narrow margin between effective and toxic doses. This article compares how the FDA, EMA, Canada, Japan, and others regulate these high-risk medications, and why global harmonization is critical for patient safety.
View MoreQuality by Design in Generic Drug Development: Modern Science-Based Approaches
By Lindsey Smith On 15 Dec, 2025 Comments (15)
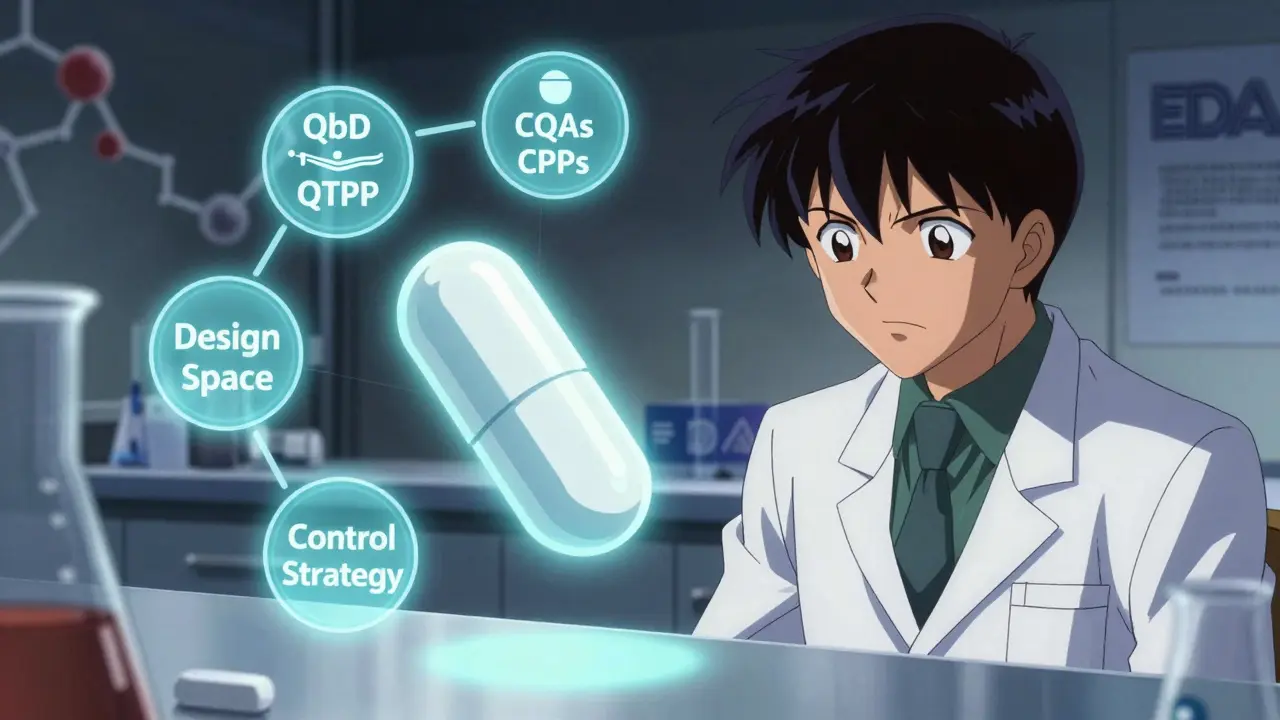
Quality by Design (QbD) transforms generic drug development by building quality into the process from the start. Learn how modern science-based approaches improve approval rates, reduce costs, and ensure bioequivalence without relying on end-product testing.
View More