Category: Pharmaceutical Information - Page 2
Insulin Therapy Side Effects: Managing Hypoglycemia and Weight Gain
By Lindsey Smith On 27 Jan, 2026 Comments (10)

Insulin therapy is essential for many with diabetes, but hypoglycemia and weight gain are common, serious side effects. Learn how to prevent lows, manage weight, and use new tools like CGMs and GLP-1 drugs to stay safe and healthy.
View MoreHow FDA Ensures Generic Drug Quality During Manufacturing
By Lindsey Smith On 24 Jan, 2026 Comments (11)
The FDA ensures generic drug quality through strict cGMP standards, unannounced inspections, and rigorous testing of every manufacturing step. Learn how the system keeps millions of Americans safe with affordable medications.
View MoreOTC Medication Expiration Dates: What Really Matters and What You Can Ignore
By Lindsey Smith On 22 Jan, 2026 Comments (14)
Expiration dates on OTC meds don't mean they're unsafe after that date-most pills stay effective for years. Learn which ones are safe to use and which ones you must replace.
View MoreFluoroquinolones and Tendon Rupture: What You Need to Know About the Risk
By Lindsey Smith On 19 Jan, 2026 Comments (13)

Fluoroquinolone antibiotics like ciprofloxacin and levofloxacin can cause sudden, serious tendon injuries-including rupture-especially in older adults and those on steroids. Know the signs, risks, and what to do if pain starts.
View MoreMarket Exclusivity Extensions: How Pharma Companies Extend Monopolies Beyond Patents
By Lindsey Smith On 18 Jan, 2026 Comments (11)

Market exclusivity extensions let drug companies block generics for years after patents expire. Learn how NCE, orphan, pediatric, and other regulatory protections create hidden monopolies in pharmaceuticals.
View MoreOpioids in Renal Failure: Safer Choices and Dosing Guidelines for Kidney Patients
By Lindsey Smith On 12 Jan, 2026 Comments (15)
Learn which opioids are safe for kidney failure patients, how to adjust doses based on kidney function, and why common painkillers like morphine and codeine must be avoided. Evidence-based guidance for safer chronic pain management.
View MoreOut-of-Pocket Costs: What Patients Pay for Generics vs Brand-Name Drugs
By Lindsey Smith On 7 Jan, 2026 Comments (12)

Generics are usually much cheaper than brand-name drugs, but insurance rules and Medicare policies can make them cost more out of pocket. Learn how your plan affects what you pay and how to save money.
View MoreHow Family History and Genetics Affect Your Response to Generic Drugs
By Lindsey Smith On 6 Jan, 2026 Comments (15)
Family history and genetics play a major role in how your body responds to generic drugs. Learn which genes affect drug metabolism, how testing can prevent side effects, and what to ask your doctor before switching to generics.
View MorePostoperative Ileus and Opioids: How to Prevent and Treat Delayed Bowel Function After Surgery
By Lindsey Smith On 5 Jan, 2026 Comments (12)

Postoperative ileus is a common, opioid-driven complication after surgery that delays recovery. Learn how multimodal pain control, early mobility, and targeted medications can prevent or treat it effectively.
View MoreAuthorized Generics: Same Drug, Different Label
By Lindsey Smith On 4 Jan, 2026 Comments (0)

Authorized generics are the exact same medication as brand-name drugs, just sold under a different label. Learn how they work, why they exist, and how they can save you money without changing the drug you're taking.
View MoreBenzodiazepines and Opioids: The Deadly Respiratory Risk
By Lindsey Smith On 3 Jan, 2026 Comments (10)
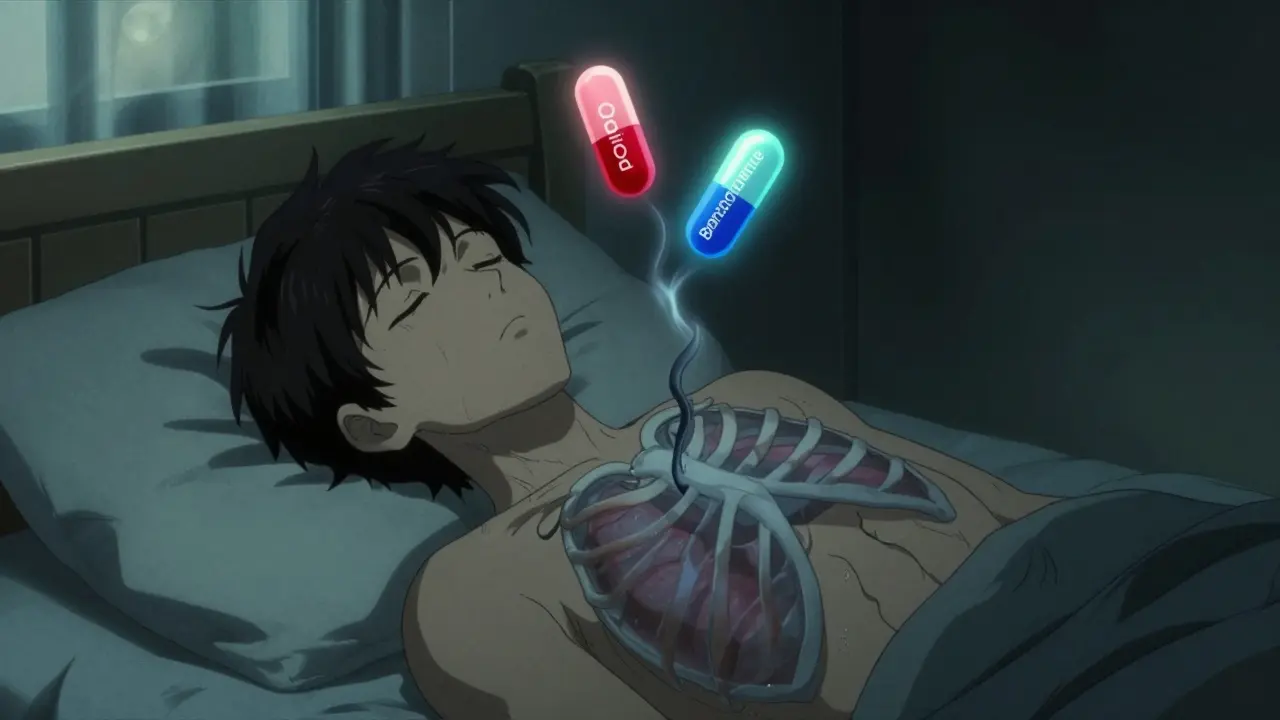
Combining benzodiazepines and opioids dramatically increases the risk of fatal respiratory depression. This deadly interaction is responsible for thousands of overdose deaths each year-and it's entirely preventable.
View MoreGeneric Drug Contamination Risks: How to Prevent and Respond to Unsafe Medications
By Lindsey Smith On 31 Dec, 2025 Comments (12)

Generic drugs save money but carry hidden contamination risks from global supply chains. Learn how contamination happens, who's at risk, and what patients and pharmacists can do to stay safe.
View More




